Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate.
In this case, fractional distillation is used in the separation of different gases in their liquid state.
Fractional distillation of air requires two steps. First, the air is cooled to a very low temperature, turning it into a liquid. Second, the liquid is heated, which allows each gas with the liquid air to evaporate at different temperatures, since each gas has a different boiling point.
Apparatus:
-Fractional column
-Cooler
-Pump
-Heater
-Expansion chamber (cooling of the air)
Example:

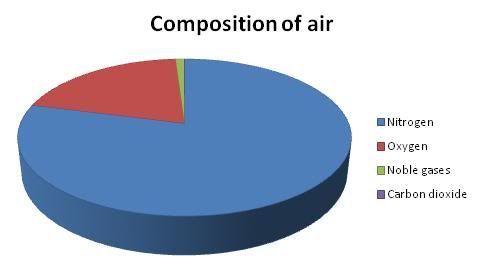
The key to success here is that every element within air has its own unique boiling point. As long as we know these boiling points, we will know when to collect each gas.
Before the liquefied air is pumped into the fractionating column, solid water and CO2 is first removed as they are both solids at -100°C. Air is then compressed to about 200 atm(atmosphere) and cooled to around -200°C. The fractional column is slowly heated by a heater and the air slowly evaporates.
From the diagram above, you can see that the gases with lower boiling points will be collected at the higher part of the column and the gases with a slightly higher boiling point is collected at the lower parts of the column.
With this, the different gases would be separated efficiently.
Credits : www.science-facts.com
www.ehow.com